A new paradigm for the treatment of acute clots
Focusing on a key driver of pathological clotting through a targeted, reversible approach
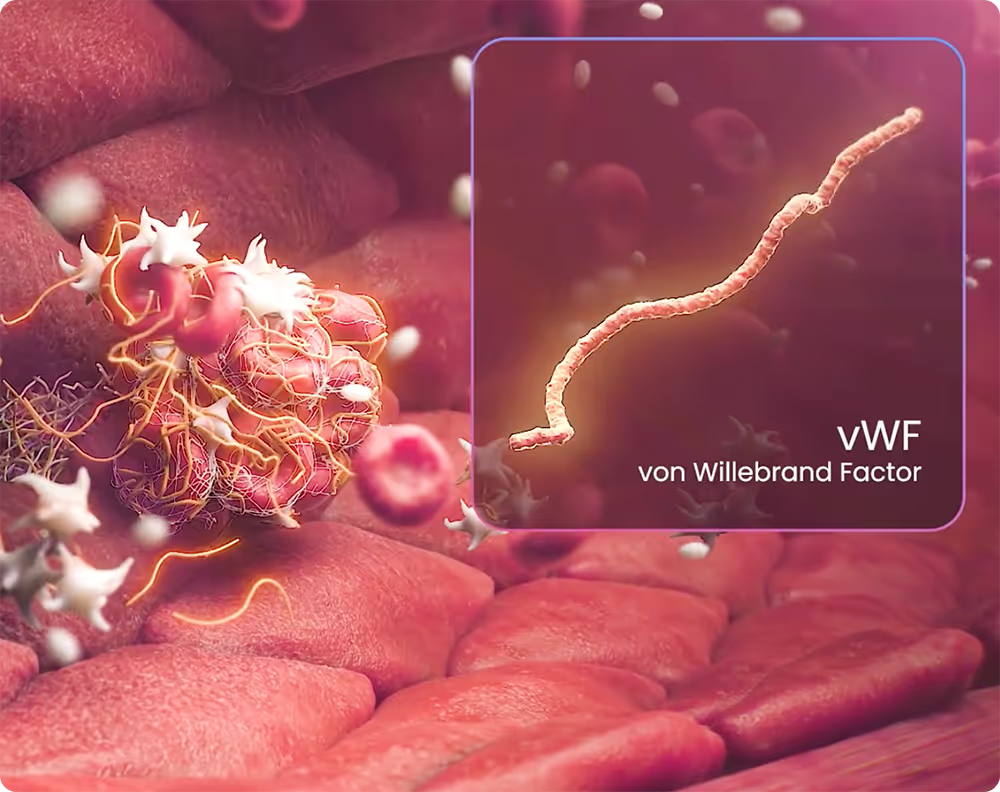
Our work is rooted in a deep understanding of the biology behind clot formation. By targeting von Willebrand Factor (vWF), a key mediator of clot initiation, growth and stabilization, we are advancing a safer and more effective treatment for acute thrombosis.
EXPLORE THE SCIENCE